INNOVATION
THAT GOES
BEYOND
HEALING
Qcil Marks 20 Years with Groundbreaking for New Factory


WHO WE ARE
Made in Africa, for Africa
Life after well
Quality Chemical Industries Limited (Qcil) is a Pharmaceutical Company established in 2005 in Kampala, Uganda. The Company is committed to prioritising Availability, Accessibility & Affordability of medicines by manufacturing in Africa.
At Qcil, we believe that health is not merely the absence of illness but the vibrant pursuit of life's possibilities. In Africa, we celebrate vitality and resilience – which is why, for us, it's about thriving, not surviving. Our belief makes us more than manufacturers of quality, affordable medications; we are enablers of “Life after well”.


OUR PRINCIPLES

Vision
To become a centre of excellence in the manufacturing of quality, affordable medicines.

Mission
To improve the quality of life by increasing access to effective, affordable medicines.

Values





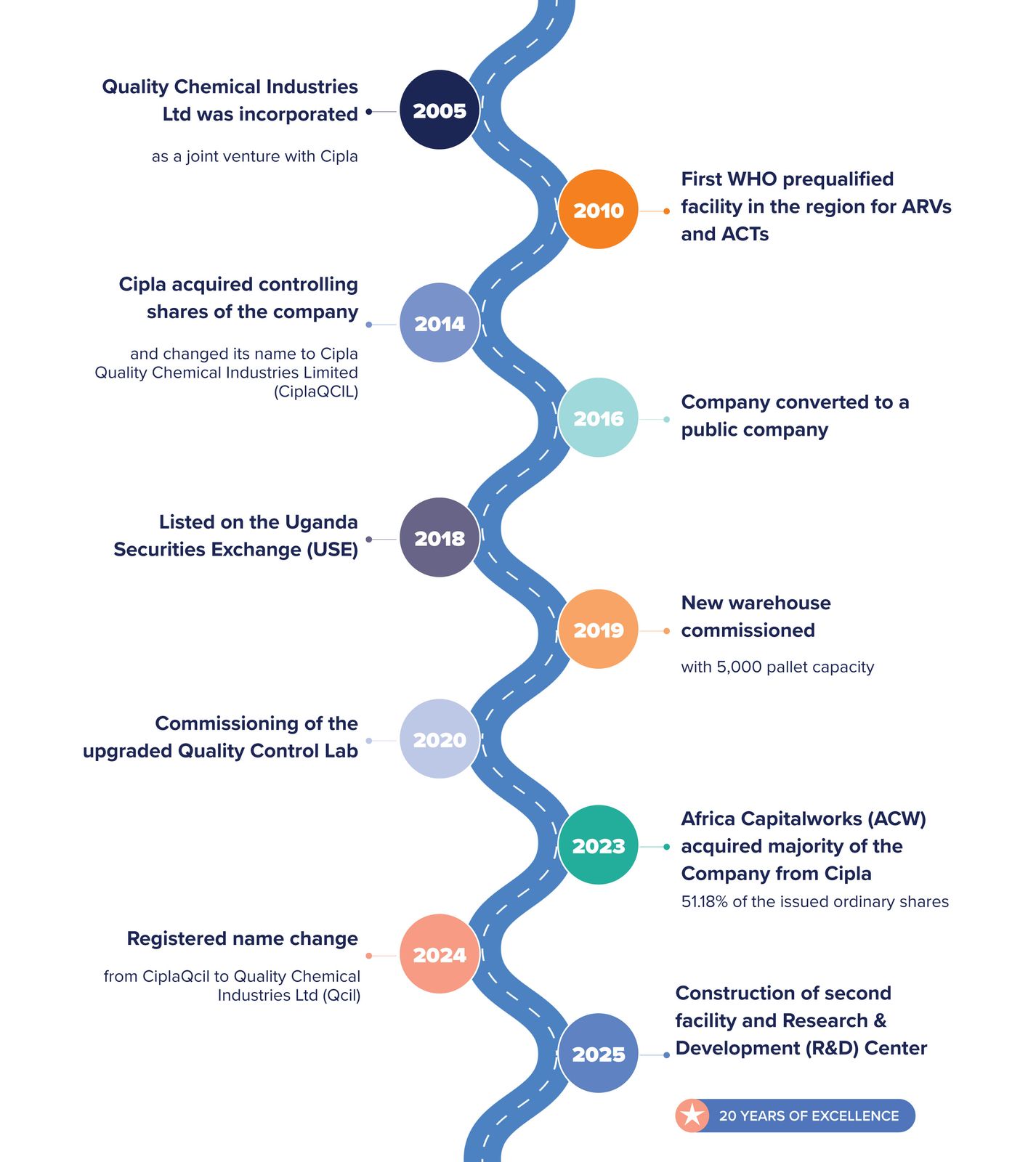
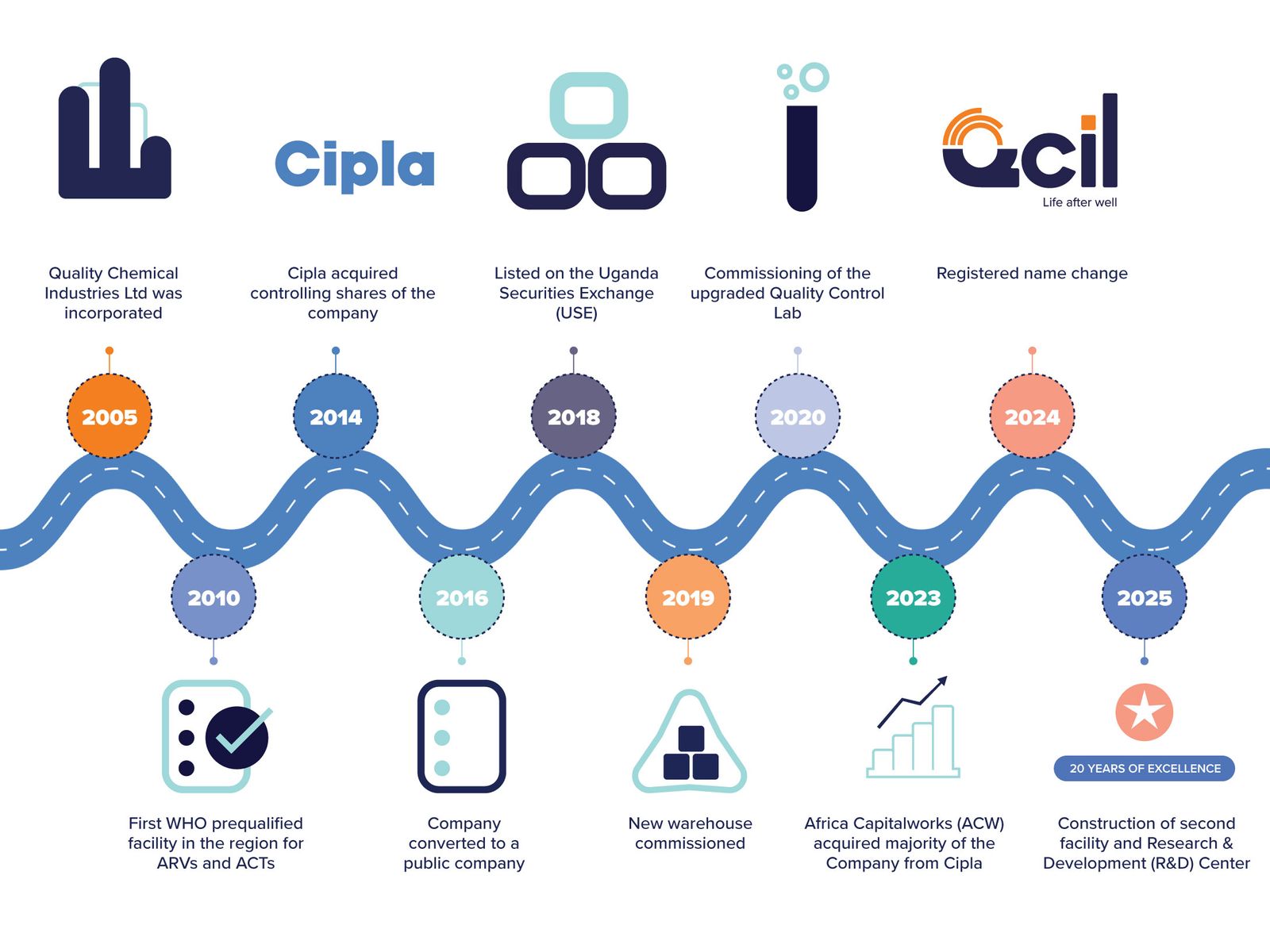
OUR JOURNEY
SUSTAINABILITY
We have developed a framework, AIR, to drive sustainable growth while producing high-quality, affordable medicines in Africa for Africa. It aligns with global standards such as the UN SDGs, GRI, IFRS S1/S2, and SASB, while reflecting Qcil’s unique identity and purpose.
Access with Integrity: Guaranteeing equitable access to essential medicines while upholding the highest ethical standards.
Identity with Impact: Strengthening our brand through social responsibility and community engagement.
Resilient and Responsible Operations: Embedding safe, sustainable practices that protect employees, communities, and the environment.
Just as clean air sustains life, AIR symbolises our commitment to people, planet, and performance, ensuring growth with integrity, purpose, and long-term impact.
Our sustainability strategy is anchored in our mission to provide affordable and effective medicines, while improving the quality of life in the communities in which we operate. The strategy focuses on two main objectives: reducing the environmental impact of our operations and contributing to the shared vision of our society.
CARBON
EMISSIONS
In respect of Scope 1 emissions, we are aspiring to achieve carbon neutrality.

LAND
POLLUTION
We are focused on reducing waste generated by our operations that goes to landfill, and we aspire to achieve zero waste to landfill.


WATER
CONSUMPTION
We are committed to reducing our consumption of blue water and aim to achieve water neutrality (Blue).

WASTEWATER TREATMENT
Qcil is dedicated to meeting safe discharge targets established by the Antimicrobial Resistance Industry Alliance, ensuring responsible wastewater management.
OUR COMMITMENT TO EXCELLENCE



Quality
We are passionate about excellence and committed to providing the highest standard of products to our customers. Quality is embedded in every step of our operations, including procurement, manufacturing, packaging, testing, distribution, sales and safe product disposal. Our management systems ensure this excellence, guided by our quality policy and manual, which cover four key areas:
Process performance and product quality monitoring.
Corrective and preventive action.
Change management.
Management review of process performance and product quality.
Our focus is on enhancing our quality management systems to meet and exceed the expectations of regulatory authorities such as WHO, NDA, SAHPRA, ZAZIBONA, EAC and COMESA. We have digitised our quality management systems using applications like Trackwise. We recognise that adequate and timely investment in continuing to strengthen these systems will enhance stakeholder confidence and bolster compliance.
We have state-of-the-art manufacturing facilities that are cGMP-compliant, adhering to both national and international standards. Additionally, our quality control laboratories use the Laboratory Information Management System (LIMS), which significantly
enhances compliance in data management. Our facilities ensure quality through regular equipment upgrades, the adoption of technological advances, and the implementation of industry benchmarked practices.
Pharmacovigilance
Pharmacovigilance is an essential component of our quality management, enabling us to adopt adequate measures to identify and raise awareness about the adverse effects of our products. Our system is equipped to handle and process safety-related complaints efficiently. Through a structured customer complaint redressal system, drug safety complaints are directed to our pharmacovigilance team.
The pharmacovigilance team also undertakes measures to review current literature to understand evolving drug reactions. The redressal mechanism is supported by a dedicated phone line and mailbox, where safety-related complaints are received from consumers, patients, and healthcare professionals. We have an established Standard Operating Procedures for diligent follow-up with each reporter. As part of this follow-up, we inquire about the patient’s safety and obtain adequate information to assess the drug’s safety profile.
IMPACT ON SDGs
At Qcil, stewardship is integral to our Company strategy. We are committed to ensuring that every aspect of our business activities aligns with our sustainability agenda. Our dedication to Environmental, Social and Governance (ESG) principles is evident through the active support and leadership of our Board of Directors and Board-level committees. The Board has clearly defined our ESG priorities, set strategic direction, and is responsible for monitoring our ESG progress. We are also committed to advancing the Sustainable Development Goals (SDGs), a global framework for promoting a more responsible future. By aligning our ESG strategy with the SDGs, we ensure that our business activities contribute meaningfully to these global goals.
Specifically, our impact is reflected through six SDGs that we have identified as material to the success of our business operations, namely:






OUR CAPABILITIES

Manufacturing

Packaging

Testing

Storage

Sales & Distribution
More than 450 employees
More than 1.4 billion tablets and hard gelatin capsule units/year
Additional warehouse capacity with more than 5000 pallets
FACTORY TOUR
Step inside our state-of-the-art facility and see how we’re revolutionising healthcare.
Watch now to witness innovation in action!

REGULATORY APPROVALS








ISO Certified
We have implemented a robust Environmental, Health and Safety Management System (EHS MS) based on ISO standards, which operates as an integral part of our business activities and encompasses manufacturing, importation, sales and distribution of pharmaceutical products. In 2023, an annual surveillance and recertification audit was conducted by third-party auditors and no significant non-conformances were reported.
REGULATORY BODIES THAT HAVE
APPROVED OUR SITE/PRODUCTS

Our Leadership
Board of Directors

MR EMMANUEL KATONGOLE
CO-FOUNDER & CHAIRMAN
Mr Emmanuel Katongole is a Co-founder of Quality Chemical Industries Limited (Qcil) and was appointed Chairman in November 2013 after serving as Founder CEO of the company from 2005. He also serves as Chair of the Nominations Committee. Previously, Emmanuel served as Managing Director of Quality Chemicals Limited (QCL), a pharmaceutical distribution company concentrating on animal health. He has a wealth of experience in corporate governance, strategy and business entrepreneurship. Emmanuel holds a Master of Arts Degree in Economic Policy and Planning and a Bachelor of Statistics Degree, both from Makerere University.
Emmanuel is also a member of the Initiative for Global Development (Frontier 100) – a group that brings the most successful business leaders operating in frontier markets together. He is the former Chairman of the Uganda National Oil Company, the former Chairman of Mauritius Union Assurance (U) Ltd, a former Board Member of Absa Bank (U) Ltd and Chairman of the Advisory Board of London-based TLG Capital Ltd.
In April 2020, he was appointed by His Excellency the President of Uganda to chair the National Response Fund on Covid-19 Taskforce in Uganda. In February 2021, he was installed as the 5th Chancellor of Nkumba University in Uganda and was appointed as a Papal Knight by Pope Francis in June 2021.
He was the East African Winner and representative at the 2013 Ernst and Young World Entrepreneur of the Year Awards in Monte Carlo where he was inducted in the prestigious World Entrepreneur of the Year Hall of Fame, received the 2012 Africa Business Leadership and the Africa Entrepreneurship Awards, and was a finalist for the East Africa Ernst and Young Entrepreneur of the Year 2011 Awards. He is a Rotarian who has steadily and diligently served his Rotary Club of Muyenga as President, Uganda Country Chairperson for Annual Giving, Assistant Governor, Chairperson of the 2010/11 Rotary District Conference and was the first District Governor for Rotary District 9211, which comprised Tanzania and Uganda in 2013-14. He has further served on committees at both Rotary Africa zone and at Rotary International.

MR FREDERICK MUTEBI KITAKA
CO-FOUNDER & DIRECTOR
Mr Frederick Mutebi Kitaka is a Co-Founder and was the Chief Finance Officer at Qcil, from 2000 to 2005.
Frederick is a Ugandan entrepreneur, industrialist and philanthropist, with specific experience in pharmaceutical manufacturing and real estate development. He has extensive expertise in financial planning and management, economic policy, investment management and corporate governance. He holds a BSc in Accounting and Finance from the University of Buckingham (UK) and a BSc in Physics and Mathematics from Makerere University.

MR GEORGE BAGUMA
CO-FOUNDER & DIRECTOR
Mr George Baguma is a Co-Founder and was the Chief Commercial Officer & Director of Marketing at Qcil, from 2000 to 2005.
George is a former Deputy Commissioner at the Directorate of Animal Resources in the Ministry of Agriculture, Animal Industries and Fisheries in Uganda. He has over two decades of expertise in the human, animal, agriculture and public health industries, in both technical and marketing capacities. He holds a Master’s degree from Imperial College in London, a Postgraduate Diploma and a Bachelor of Science (Honours) from Makerere University.

MR AJAY KUMAR PAL
CHIEF EXECUTIVE OFFICER
Mr Ajay Kumar Pal has served as the Chief Executive Officer of Qcil since August 2021. He has worked in the pharmaceutical industry for over two decades and has held various executive positions in major pharmaceutical companies. His career spans across three countries – Uganda, South Africa and India.
Ajay holds an MBA from Nelson Mandela University Business School in South Africa and a Bachelor of Pharmacy, Industrial and Physical Pharmacy, and Cosmetic Sciences from Rajiv Gandhi University of Health Sciences in India.
Ajay's expertise include operations management, business transformation, acquisitions, maximising business profitability, strategic planning, leadership and technology transfer. Throughout his career, Ajay has prioritised bold transformation within organisations, and has successfully led and motivated teams in this respect. He served as General Manager and Senior Director at Cipla Medpro Manufacturing company in South Africa, after its acquisition by Cipla in 2013, during which time he led the technology transfer of 18 new products, facilitated the company's return to profitability and the company’s acquisition of Mirren Proprietary Limited in 2018.
Ajay joined Qcil as Chief Operating Officer in February 2020. Under his leadership, the Company has increased its manufacturing and distribution capacity, extended its product portfolio and originated initiatives related to healthcare access, environmental sustainability and ethical practices.
He is currently serving as a member of the Audit and Risk Committee.

MRS BETH MANDEL
NON-EXECUTIVE DIRECTOR
Mrs Beth Mandel is a Co-founder and Managing Partner of Africa Capitalworks and Capitalworks Investment Management, a position she has held since 2007. She also previously held positions as a Managing Director and Country Head SSA of Morgan Stanley and Founding Director of RMB Morgan Stanley.
Beth holds a Master of Science in Development Economics from New College, Oxford University (Marshall Scholar) and a BSc with high honours in Business Administration (Production Management).
She has extensive global experience in corporate finance, mergers and acquisitions, strategy and investment management.
She is currently serving as a member of the Audit and Risk Committee and Remuneration Committee.

MR JOSEPH BALIDDAWA
INDEPENDENT NON-EXECUTIVE
Mr Joseph Baliddawa is a former partner of PricewaterhouseCoopers Africa (PwC) and Country Senior Partner for PwC (Uganda) having spent over three decades with PwC in a variety of roles in the UK, Zambia and Uganda.
Joseph is a Fellow of the Association of Chartered Certified Accountants (FCCA), a Member of the Institute of Certified Public Accountants of Uganda (CPA) and a Founder Council Member of both the Institute of Certified Public Accountants of Uganda and the Zambia Institute of Chartered Accountants (ZICA). He has extensive experience in management and leadership, having served in PwC’s country management, formulation and monitoring of the implementation of strategic growth plan for Africa, risk, quality and compliance standards management and financial reporting.
He is a former President of the Institute of Corporate Governance of Uganda and is currently a Board member and the Chair of the Audit Committees of the NCBA and Alliance Africa General Insurance Limited.
He currently serves as Chair of the Audit and Risk Committee and is a member of the Remuneration Committee.

DR PETER MUGYENYI
INDEPENDENT NON-EXECUTIVE
Dr Peter Mugyenyi is a holder of a Doctor of Science (ScD(h)) and a Bachelor of Medicine and Surgery (MB ChB). He is also a Fellow of the Royal College of Physicians of Ireland (FRCPI) and a Fellow of the Royal College of Physicians (Edinburgh) (FRCP Edin).
Peter is a paediatrician, researcher and specialist on HIV/AIDS and related conditions. His research and publications cover a wide spectrum of subjects within this field, including paediatric and adult trials, HIV resistance, HIV prevention, immunological studies including HIV vaccine trails, pharmacokinetic, molecular and epidemiological studies, as well as the social and economic impact of HIV.
He was among the pioneers who introduced the widespread use of affordable ARVs in Africa, as well as the development of an effective model of ARVs in Africa and the development of an effective model for scaling up ARVs in resource-limited countries. He has also been a Principal Investigator on multiple landmark research projects funded by National Institute of Health (NIH), the European Union, WHO and the Medical Research Council (MRC).
He has served as a Board member on several institutions and organisations in Africa, UK, India and the USA. Until his retirement, he was the Executive Director of the Joint Clinical Research Centre.
He is currently serving as the Chair of the Remuneration Committee.

MR STEVENS MWANJE
NON-EXECUTIVE DIRECTOR
Mr Stevens Mwanje is the CFO of the National Social Security Fund (NSSF) and a Fellow of the Association of Chartered Certified Accountants (FCCA).
He holds a Master’s in Business Administration from Edinburgh Business School – Herriot Watt University, a Postgraduate Diploma from the University of Leicester and a Postgraduate Diploma in Business Management from Uganda Management Institute. He undertook the Strathmore Business School Executive Programme. He has also completed several short courses on leadership, risk management, finance management, performance management and corporate governance.
Previously, he served as the Head of Sales and Operations at NSSF, Head of Commercial Decisions – Bank of Africa, Chief Accountant and Head of Internal Controls at Allied Bank International, Uganda. He is currently the Chairperson of the Board Audit Committee of the Entrepreneurs Financial Centre (EFC) and serves on the Board of Kampala Club Limited.
He is also a member of the Rotary Club – Kampala North.
He is currently serving as a member of the Audit and Risk Committee.

MR VUSI RASEROKA
NON-EXECUTIVE DIRECTOR
Mr Vusi Raseroka is an accomplished and highly experienced private equity investment professional with over three decades of experience in financial services, corporate finance, financing and investments across the African continent. He is passionate about investing in Africa and has transacted across multiple jurisdictions on the African continent. He is an Unlisted Investments Specialist and Fund Manager with extensive private equity, infrastructure and project finance (equity and debt) knowledge, skill and expertise.
Vusi is employed by the Public Investment Corporation (PIC) SOC Limited as a Fund Principal in the Unlisted Investments Division, where he heads the Developmental Investments Fund for Africa ex SA. Prior to this, he was a Portfolio Manager – Economic Infrastructure. Thereafter, he became an Associate Fund Principal and then a Fund Principal in the Private Equity division, all within the PIC. Prior to joining the PIC, Vusi was employed by the Development Bank of Southern Africa (DBSA), in the International Finance Division.
Prior to joining the DBSA, he was the Head of Privatisation and Restructuring at the Public Enterprises Evaluation and Privatisation Agency in Botswana. Prior to this, he held various banking and corporate finance roles in Botswana.
Vusi holds a BCom degree in Accounting from the University of Botswana, is a Fellow of the Association of Chartered Certified Accountants (FCCA) (UK) and is a member of the Institute of Directors of Southern Africa.
He is currently serving as a member of the Remuneration Committee.

DR FRANCES PHILOMENA NAMATOVU
EXECUTIVE DIRECTOR
Dr. Namatovu is a seasoned pharmacist with over 10 years of experience in the pharmaceutical industry.
She currently serves as the Head of Regulatory Affairs and Pharmacovigilance at Quality Chemical Industries Limited, where she plays a key role in regulatory compliance, drug safety, and strategic business development.
Her distinguished career encompasses leadership roles at Quality Chemical Industries Limited, where she has played a pivotal role in guiding the Company through intricate regulatory and business challenges within the East African Community, Southern African Development Community, and the Economic Community of West African States.
Dr. Namatovu holds a Master of Science in Drug Development with Biobusiness from Aberdeen University, Scotland and a Bachelor of Pharmacy from Manipal University, Karnataka, India. She is also a member of the Pharmaceutical Society of Uganda.
Management Team

MR AJAY KUMAR PAL
CHIEF EXECUTIVE OFFICER
Mr Ajay Kumar Pal has served as the Chief Executive Officer of Qcil since August 2021. He has worked in the pharmaceutical industry for over two decades and has held various executive positions in major pharmaceutical companies. His career spans across three countries – Uganda, South Africa and India.
Ajay holds an MBA from Nelson Mandela University Business School in South Africa and a Bachelor of Pharmacy, Industrial and Physical Pharmacy, and Cosmetic Sciences from Rajiv Gandhi University of Health Sciences in India.
Ajay's expertise include operations management, business transformation, acquisitions, maximising business profitability, strategic planning, leadership and technology transfer. Throughout his career, Ajay has prioritised bold transformation within organisations, and has successfully led and motivated teams in this respect. He served as General Manager and Senior Director at Cipla Medpro Manufacturing company in South Africa, after its acquisition by Cipla in 2013, during which time he led the technology transfer of 18 new products, facilitated the company's return to profitability and the company’s acquisition of Mirren Proprietary Limited in 2018.
Ajay joined Qcil as Chief Operating Officer in February 2020. Under his leadership, the Company has increased its manufacturing and distribution capacity, extended its product portfolio and originated initiatives related to healthcare access, environmental sustainability and ethical practices.
He is currently serving as a member of the Audit and Risk Committee.

DR FRANCES PHILOMENA NAMATOVU
HEAD OF REGULATORY AFFAIRS AND PHARMACOVIGILANCE
Dr Namatovu oversees Regulatory Affairs and Pharmacovigilance for Qcil.
She is a Regulatory Affairs professional with over a decade of experience in diverse regulatory environments across Africa (East African Community, Southern African Development Community, Economic Community of West African States) and the World Health Organization (WHO).
She holds a Master of Science Degree in Drug Development with Bio Business, from Aberdeen University – Scotland, UK and a Bachelor of Pharmacy degree from Manipal University, Karnataka – India. She is a member of the Pharmaceutical Society of Uganda.

MR FRED ANDREW KAKOOZA
CHIEF FINANCE OFFICER
Mr Kakooza joined CiplaQCIL in March 2020 and oversees the finance, tax and ICT functions of the company. He was previously the Head of Finance at Kampala Pharmaceuticals 1996 Limited (KPI). Prior to working at KPI, he was the Chief Finance Officer and Financial Management Expert at Energy Utility Corporation Limited, Rwanda’s national electricity distributor. He has also held senior roles at Umeme Limited, Warid Telecom Uganda Limited and MultiChoice Uganda Limited. He started his career at Deloitte & Touche (Deloittes). He has over two decades of experience in Uganda and Rwanda, where he has built a strong background in strategic performance management and streamlining robust operations across the telecoms, utilities and manufacturing sectors.
Mr Kakooza holds a Master of Business Administration Degree from Heriot-Watt University in the UK and a Bachelor of Science Degree in Economics and Statistics from Makerere University. He is a Fellow of the Association of Chartered Certified Accountants (FCCA), Fellow of Chartered Institute of Management Accountants (FCMA) and a Member of the Institutes of Certified Public Accountants of Kenya and Uganda.

MR ATUL VADEPALI
HEAD OF QUALITY CONTROL AND QUALITY ASSURANCE
Mr Vadepali has been the Quality Assurance Manager at CiplaQCIL since 2007. Prior to this, he worked for Cipla India in the Quality Assurance department as of 2002. He has over a decade of experience in pharmaceutical quality assurance and holds a Bachelor’s Degree in Pharmacy, from Pune University in India.

MR HARRISON KIGGUNDU
HEAD OF HUMAN RESOURCES
Mr Kiggundu has been the Human Resources Manager at CiplaQCIL since 2009. Prior to this, he worked for Uganda Breweries Limited, first as a brewer and then rose through the ranks to the level of Packaging Manager, Capability Development Manager and Human Resources Manager. Now with over two decades of experience, he holds a Bachelor of Science in Food Science and Technology from Makerere University, a Diploma in Brewing from Herriot Watt University in the UK, a Postgraduate Diploma in Human Resources and a Master’s Degree in Human Resources Management, both from the Uganda Management Institute (UMI).

MS GRACE KARUHANGA
COMPANY SECRETARY & HEAD OF LEGAL AFFAIRS
Ms. Grace Karuhanga is a seasoned legal professional with extensive experience in both Private and Public Sectors. She specializes in legal advisory and compliance, driving organizational success through fostering transparency, good governance, and optimized corporate operations. Her expertise includes board management, legal document drafting, corporate governance guidance, and enhancing corporate reputation.
She holds an MBA from the University of Leicester, a Masters Degree in Commercial and Corporate Law from the University of London, a Diploma in Legal Practice from the Law Development Centre, a Law degree from Makerere University, and a certificate in Institute of Chartered Secretaries and Administrators, UK.
Her career spans roles such as Coordinator of the Investment Advisory Committee at the Ministry of Finance, Planning and Economic Development, and Head of Legal and Company Secretary at Guaranty Trust Bank (U) Ltd. She has also held Legal positions at Bayport Financial Services, Global Trust Bank and Standard Chartered Bank (U) Ltd.

MS SARAH MUSUMBA
HEAD OF ESG
Ms Musumba is a seasoned sustainability and ESG executive with over a decade of experience in both the public and private sectors. She is passionate about driving positive social and environmental impact, while advancing business objectives. She has a proven ability to develop and execute sustainable strategies that create social value, improve environmental performance and save money. As an ESG Executive, she is passionate about using the power of business, to solve social and environmental challenges.
As the ESG Lead at Qcil, Ms Musumba is tasked with leading the development and implementation of the Company’s ESG strategy, in line with the Company’s strategic focus. She also oversees the ESG reporting and disclosures process, ensuring transparency and accountability in the company’s sustainability performance. Key to integrating sustainability considerations into business operations, has been a collaboration with cross functional teams, product development and supply chain management. Relatedly, she spearheads stakeholder engagement initiatives, partnerships with NGOs and Government Agencies and industry stakeholders, to drive collective action on sustainability challenges.
Her key skills and expertise include: ESG strategy development, ESG integration and reporting, stakeholder engagement and partnerships, corporate social responsibility, environmental management and compliance, and cross-functional leadership and collaboration.
She also holds a Postgraduate degree in ESG and Sustainability from Wharton University of Pennsylvania, a diploma in Strategy and sustainability from IESE Business School in Spain, a diploma in Business planning and development and strategic thinking from Harvard Business School, and a Bachelor’s degree in Social Work and Social Administration, from Makerere University Kampala.
Her belief in lifelong learning and drawing inspiration from new experiences has shaped her into an avid reader across a variety of genres, with an open mind and curious approach to life.

MR PANDA RAMAKANTA
HEAD OF OPERATIONS
Mr Ramakanta was appointed Head of Operations in 2023.
Prior to his appointment, he served as Head of Manufacturing at Cipla Quality Chemical Industries since 2007 and Head of Production at Cipla India since 2003.
He has over two decades of experience in core operations, with expertise in Green Field projects, operations and technology transfer. He possesses strong leadership, product development and quality management system skills.
He holds a Bachelor’s degree in Pharmacy from Utkal University – India.

MR MAHADEV MANDHARE
HEAD OF SUPPLY CHAIN
Mr Mandhare has over a decade of experience in Supply Chain Management, with a unique blend of expertise in Procurement, Demand Planning, Supply Planning, Production Planning, Logistics, Material Planning, Warehousing, Distribution and SAP Projects, including, but not limited to, S4 HANA Implementation.
He joined Qcil in February 2023 as Head of Supply – Chain Management. Previously, he worked at Cipla Ltd as a Cluster Head – material planning and at Encube-India as Head of Planning. He delivered extraordinary results that drove organisational profitability, growth and customer satisfaction.
He holds a PGDM degree in Supply Chain Management and a Bachelor of Science in Chemistry.

MR ROHIT DATAR
HEAD OF BUSINESS DEVELOPMENT AND PRIVATE MARKET
With over two decades of experience in business development and sales operations in the pharmaceutical industry of emerging markets, Mr Datar has demonstrated proficiency in closing deals, developing ideas, managing partnerships and building high-performing teams. This is evidenced by a field-tested track record of achieving business objectives, through the planning and execution of sales and marketing strategies.
Mr Datar brings fundamental knowledge of the pharmaceutical market and the competitive landscape of finished dosage forms to the table. At Qcil, he is responsible for sharing market insights with management for planning purposes, establishing partnerships aimed at portfolio expansion, developing private market sales of the company, by offering innovative and affordable products and building a strong sales force, to enhance Qcil's presence in the private market.
Mr Datar is a commerce graduate from the University of Mumbai and holds a Postgraduate degree in marketing.
AWARDS
WINNER
Compliance & Governance Award CFO Awards 2025
WINNER
Listed Entity and Most Improved Report Categories - FiRe Awards 2025
winner
2nd runner Up Consumer & Industrial Products - Financial Reporting Awards (2025)
Winner
ASA Awards - Finance Team of the Year (2025)
WINNER
National Environment Sustainability Award for Exceptional Management of Active Chemicals - NEMA (2024)
Winner
Federation of Uganda Employer Award for Disability Inclusion (2023)
Winner
Outstanding Performance in Occupational Safety & Health Management (2023)
Winner
Best Practices in Learning and Development (2022 Survey)
Winner
Employer of the Year Award (2021/22 Survey)
Winner
Best Reward and Recognition Award - Prudential Best HR Practices (2021 Survey)
Winner
Financial Reporting Award - Fire Awards (2021)
Winner
Indian Business Forum Award - Contribution Towards the Pharmaceutical/Health Sector (2018)
Winner
Africa Award for Entrepreneurship - Transformational Business of the Year (2012)
Winner
Outstanding Achievement in Development of the Pharmaceutical Sector - Ministry of Health (2012)
Winner
Investor of the Year - Uganda Investment Authority (2007)
